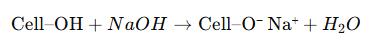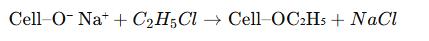Ethyl cellulose (EC) is a widely used cellulose derivative in pharmaceuticals, food, coatings, inks, and plastics. It is obtained by replacing the hydroxyl groups of the cellulose backbone with ethoxy groups through an etherification process. This chemical modification makes cellulose, which is naturally hydrophilic and insoluble in most organic solvents, into a thermoplastic polymer soluble in various organic solvents, with increased hydrophobicity and film-forming ability.

Raw Materials for Ethyl Cellulose
Cellulose – The main raw material, usually purified from wood pulp or cotton linters. High-purity cellulose with minimal lignin, hemicellulose, and ash content is required for consistent reaction efficiency.
Alkali (NaOH) – Used for alkalization, converting cellulose into alkali cellulose. Sodium hydroxide swells the cellulose structure, activating hydroxyl groups for etherification.
Ethylating agent – Typically ethyl chloride (C₂H₅Cl) or ethyl sulfate (C₂H₅OSO₃H), which reacts with alkali cellulose to substitute hydroxyl groups with ethoxy groups.
Organic solvents – Xylene, benzene, or toluene can be used as reaction media. Modern processes often use greener alternatives.
Water – Required for washing and neutralization steps.
Acids – Dilute hydrochloric acid or acetic acid is used for neutralization after the reaction.
Chemical Basis of the Process
Cellulose consists of repeating β-D-glucose units linked by β-1,4-glycosidic bonds, each glucose ring containing three hydroxyl groups (-OH) at the C-2, C-3, and C-6 positions. These hydroxyl groups are the reactive sites for etherification.
The preparation of EC involves two main chemical reactions:
Alkalization:
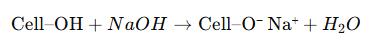
Sodium hydroxide converts free hydroxyl groups into alkoxide ions, increasing nucleophilicity.
Ethylation:
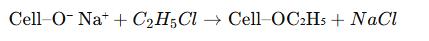
The alkali cellulose reacts with ethyl chloride (or ethyl sulfate), producing ethyl cellulose and sodium chloride as by-products.
The degree of substitution (DS), i.e., the average number of hydroxyl groups replaced by ethoxy groups per glucose unit, usually ranges between 2.2 and 2.6 for commercial ethyl cellulose. The DS significantly influences the solubility, viscosity, and film-forming properties of the polymer.
Step-by-Step Preparation Process
3.1. Preparation of Alkali Cellulose
Cellulose drying: The purified cellulose is dried to a moisture content of around 5–10% to ensure controlled reaction.
Alkalization: The dried cellulose is treated with an aqueous sodium hydroxide solution (typically 18–30% w/w) at temperatures between 25–50 °C. The NaOH penetrates cellulose fibers, converting hydroxyl groups into sodium cellulose (alkali cellulose).
Aging: The alkali cellulose is allowed to age for several hours. During aging, the crystalline structure of cellulose swells, and the polymer chains become more reactive.
3.2. Etherification (Ethylation)
The alkali cellulose is transferred to an etherification reactor.
Ethyl chloride is introduced, either in liquid or gaseous form, under pressure (1–3 MPa). Reaction temperatures typically range between 60–120 °C.
The reaction may last from 3 to 10 hours, depending on the target DS, particle size, and agitation.
Sometimes a mixed solvent (e.g., toluene with ethanol) is used to facilitate heat transfer and improve diffusion of the ethylating agent.
By-products, such as sodium chloride, are generated and remain in the reaction mass.
3.3. Product Isolation and Purification
After the reaction, the crude ethyl cellulose is separated from by-products.
It undergoes repeated washing with hot water to remove salts (mainly NaCl) and residual alkali.
Neutralization with dilute acid ensures complete removal of sodium hydroxide residues.
The washed EC is further washed with organic solvents to remove unreacted ethyl chloride or side products.
3.4. Drying
The purified wet EC is dried in hot-air dryers or vacuum ovens at temperatures around 70–80 °C until moisture content falls below 1%.
Special care is taken to prevent degradation or discoloration during drying.
3.5. Milling and Sieving
Dried EC is milled to the desired particle size.
Sieving ensures uniformity of particle distribution, important for consistency in end-use applications such as tablet coating or film casting.

Factors Affecting Ethyl Cellulose Quality
Degree of substitution (DS): Determines solubility and physical properties. Lower DS (<2) leads to partial water solubility; higher DS (>2.6) improves solubility in organic solvents but may affect mechanical properties.
Viscosity: Controlled by the molecular weight of the cellulose source and reaction conditions. EC is commercially available in various viscosity grades, important for applications in coatings and pharmaceuticals.
Purity: Residual salts, unreacted chemicals, or by-products can compromise quality, film transparency, and biocompatibility.
Moisture content: Excess moisture can reduce storage stability and affect flowability.
Environmental and Safety Considerations
Use of greener solvents: Modern production focuses on minimizing the use of aromatic hydrocarbons and using closed systems to reduce emissions of ethyl chloride.
Effluent treatment: Washing steps generate wastewater containing salts and organic residues, requiring treatment before disposal.
Worker safety: Ethyl chloride is flammable and toxic; thus, reactors are designed for high safety standards.
Applications of Ethyl Cellulose
Pharmaceuticals: Used as a film-former in tablet coatings, sustained-release matrices, and taste-masking agents.
Food industry: Serves as a stabilizer, thickener, and encapsulating agent.
Coatings and inks: Provides moisture resistance and flexibility.
Plastics: Functions as a binder and modifier in thermoplastic formulations.
Ethyl cellulose is prepared by the etherification of cellulose with ethyl chloride in the presence of alkali. The process involves several well-controlled steps: alkalization, ethylation, washing, drying, and milling. By adjusting the degree of substitution and molecular weight, manufacturers can tailor EC for specific applications. The preparation process emphasizes purity, environmental safety, and efficiency, making ethyl cellulose one of the most versatile cellulose derivatives in industrial and pharmaceutical use today.
 English
English 日本語
日本語 français
français Deutsch
Deutsch Español
Español italiano
italiano русский
русский português
português العربية
العربية Türkçe
Türkçe Nederland
Nederland